Sodium-ion batteries are an increasingly competitive area of research, thanks to their potential for low cost energy storage based on abundant, non-toxic materials. While the technology does not have energy density levels comparable to lithium-ion, it still thought of as viable for stationary storage projects and other areas where size is not a primary concern.
The material has so far seen little commercial adoption, as many of the materials required are sensitive to air and therefore expensive to produce and maintain performance levels.
Now, researchers at University of Wollongong’s Institute for Superconducting and Electronic Materials (ISEM) have developed a material which is not sensitive to air, and exhibits strong cycling stability. The proof of concept cells developed by the team are described in the journal Advanced Energy Materials.
The researchers now plan to work on optimizing the material to maximize its cycle life and ease of manufacturing. “With new materials and processing techniques we can focus on further development that will pave the way for the transition to commercialisation of this exciting and much-needed alternative to lithium-ion batteries.”
ISEM researchers also published an additional paper in Advanced Energy Materials which offers a broader discussion of progress toward commercialization of sodium-ion technology, and identifying key indicators for commercial viability.
“Commercial full-cell design includes optimising capacity balancing between the cathode and the anode, finding a stable electrolyte solution, choosing appropriate additives and binders, selecting a separator, as well as the production costs of the active materials for the electrodes and the overall manufacturing cost of the batteries,” says Luo. “To a large extent, how the cycling performance, or battery lifespan, satisfies the requirements of large energy storage systems will determine its commercialisation progress. We need to develop batteries that provide a long life-span to justify the investment.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.
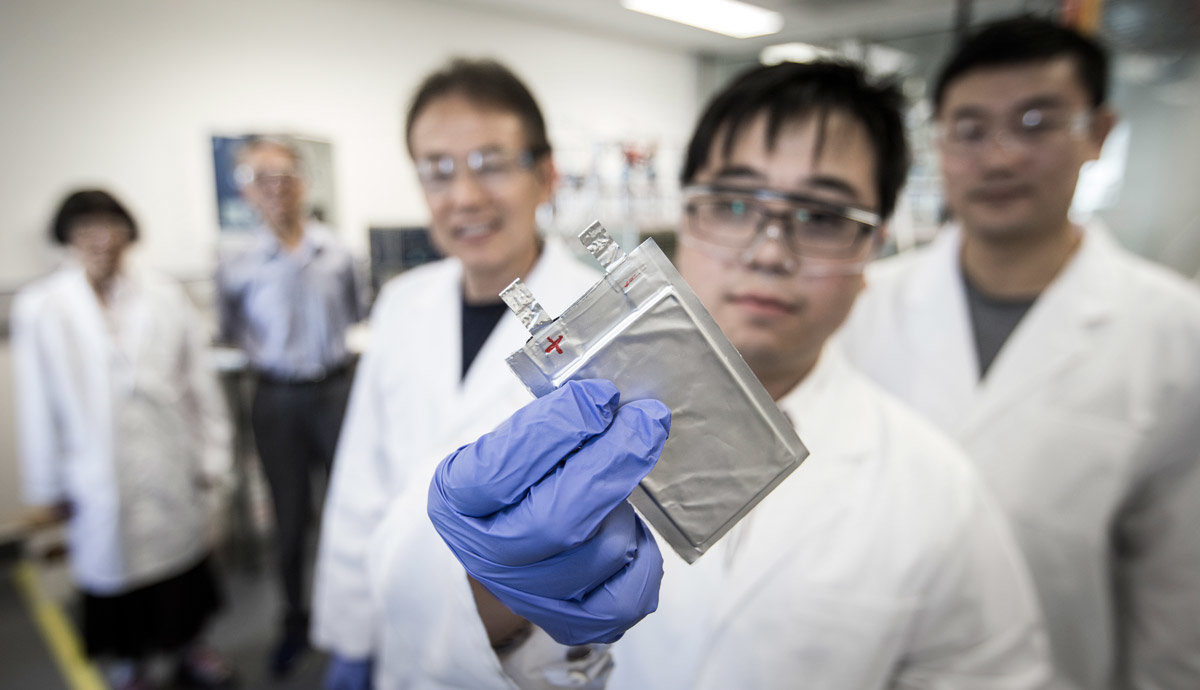
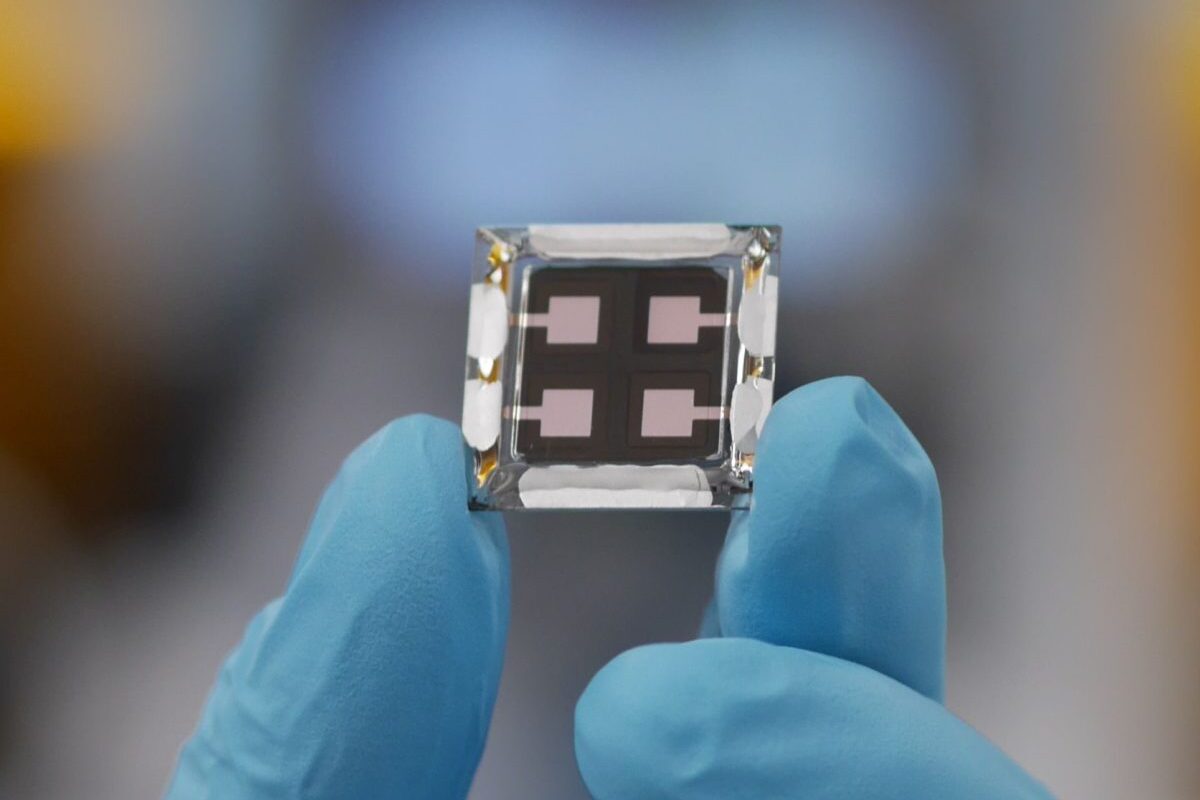







From the abstract: “It exhibits a reversible capacity of 172 mA h g−1 at 0.1 C and remarkable capacity retention of 70.4% after 1000 cycles at 20 C. More importantly, it offers good compatibility with pristine hard carbon as anode in the sodium‐ion full cell system, delivering a high energy density of up to 215 W h kg−1 at 0.1 C and good rate performance.”
The degradation of capacity is all important in the longevity and effectiveness of the chemistry used as an energy storage unit. After the 1000 cycles, does the battery still degrade at the same “rate” during the 1000 cycles or does it attenuate and take a long time for the capacity to degrade to 65%? 60%? If the chemistry is cheap enough to manufacture, one could overdesign the battery pack storage to account for degradation with cycling.