The potential safety concerns surrounding lithium-ion batteries have burst into the public eye of late with the fire at the Victorian Big Battery (VBB). However, researchers at Monash University School of Chemistry and Australian company Calix Ltd have been working on alternative solutions without such fire hazards and toxicity issues.
As the VBB fire illustrated, fire hazards in lithium-ion batteries, though relatively rare, especially when compared to combustion engines, are a pressing issue. After all, utility-scale energy storage is set to replace and grow across large sections of the grid, and a few tonnes of concrete isn’t always around to douse such resilient fires.
Monash University’s Prof. Doug MacFarlane and Dr. Mega Kar have been working with Calix Ltd on electrolyte development in the higher power batteries used for energy storage solutions and electric vehicles (EVs). And in recent research published in Advanced Energy Materials, the chemists forward “Lithium borate ester salts for electrolyte application in next-generation high voltage lithium batteries”, which is to say, a novel lithium salt that could replace the more hazardous hexafluorophosphate salt.
“The lithium salt currently being used in lithium-ion batteries is lithium hexaflurosophosphate, which poses a fire and safety hazard as well as toxicity,” said MacFarlane. “In smaller portable devices, this risk can be partially mitigated. However, in a large battery pack, such as electric vehicles and outdoor grid scale energy storage systems, the potential hazard is much intensified. Higher voltage and power batteries are also on the drawing board but cannot use the hexafluorophosphate salt.”
The study’s lead author, the Monash University School of Chemistry’s Dr Binayak Roy, stated “our aim has been to develop safe fluoroborate salts, which are not affected even if we expose them to air. The main challenge with the new fluoroborate salts was to synthesise it with battery grade purity which we have been able to do by a recrystallisation process.”
The researchers put these novel salts into a lithium-ion battery with lithium manganese oxide cathodes and were able to cycle the cell for more than 1,000 cycles, “even after atmospheric exposure,” Roy stressed, “an unimaginable feat compared to the hyper-sensitive hexafluorophosphate salt.”
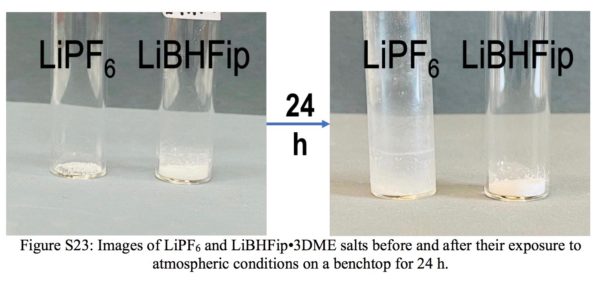
What is more, Roy said the new electrolyte, when combined with a novel cathode material, far outperformed the conventional salt, and was also found to be very stable on aluminium current collectors at higher voltages, as will be necessary for next-gen batteries.
Researchers collaborated with Calix on this study as the company produces manganese-based battery materials from Australian sourced minerals. Calix hopes this research will assist it in achieving its goal of large-scale fabrication of Australian-based lithium-ion batteries.
“Calix is developing a platform technology to produce high-performance, cost-competitive battery materials in Australia,” said Calix’s general manager for R&D, Matt Boot-Handford, “…the superior electrochemical performance and stability demonstrated by the Monash team’s new electrolyte system paired with Calix’s lithium manganese oxide electrode material is an exciting and important milestone that brings us one step closer to making batteries featuring Calix next-generation electrode materials a commercial reality.”
Monash’s Kar noted that “in the near future we hope to turn these new anions into thermally stable, non-flammable liquid salts, making them beneficial for batteries operating at high temperatures. With the current climate conditions, designing such battery technologies with safety and stability will be important in implementing a sustainable grid-scale energy solution in Australia.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.







In the context :
“lithium hexaflurosophosphate, which poses a fire and safety hazard as well as toxicity,”
I do not agree on this statement since LiPF6 (lithium hexafluorophosphate) itself does not cause any fire concern (yes, it is toxic and hazardous material). The hexafluorophosphate, which is the anion of the salt, even can extinguish flame in some point.
Hi Caleb,
Thanks for your comment. The quote you cite came to pv magazine directly from the Monash School of Chemistry, I am following up with said scientists in the context of a larger article and I’ll put your argument to them.