Solar panels are now an accepted means of producing clean electricity, and Australia is revving along the roadmap to mass production of clean hydrogen using a two-step solar conversion process — photovoltaics followed by electrolysis. But this month, Swinburne University of Technology’s Associate Professor Tianyi Ma received an Australian Research Council Future Fellowship of more than $937,000 to pursue his work using solar energy directly in photocatalysis, to repurpose abundant carbon dioxide emitted during industrial processes into “solar” fuels such as green methane, methanol and carbon monoxide.
The efficiencies of Ma’s technology are currently lower than photovoltaics which have been developed to achieve between 20% and 30% conversion of light into electricity. His work with new-generation carbon dioxide photoreduction catalysts — perovskite-based ferroelectrics — have so far achieved a solar-to-fuels efficiency of 2-5%, but that’s increasing.
Importantly, the materials used are much cheaper than those used in solar panels, offering substantial savings on upfront capital investment and the technology shows potential for co-location with industry, to siphon off CO2 at the source and produce fuels that can replace high-emitting fossil fuels.
Yes, even these solar fuels when burned will release carbon into the atmosphere, but they go some way to achieving a low-cost circular economy for carbon — capturing what’s emitted, and producing replacements for new fossil fuel production.
“My goal is carbon neutrality,” the lead of the Carbon Neutrality Group at Swinburne University of Technology tells pv magazine Australia. Boom-boom!
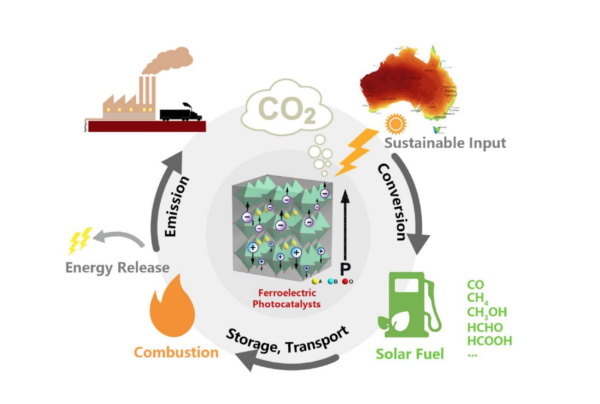
Image: Associate Professor Tianyi Ma/ Swinburne University of Technology
The drive to purity
For some years Ma was known for his work using grid-supplied electricity in a process known as electrocatalysis, for reducing CO2 into potentially reusable fuels. “The thing is,” he says, “that the majority of electricity in the grid still isn’t green, so I recently changed focus to photocatalysis, because I want to use pure renewable energy.”
Ma says his work is still “inspired” by electrocatalysis, in that he’s using an external power source to accelerate a catalytic reaction. But there’s no voltage in raw sunlight, so “if we can create a little bit of an electric field in the semiconductor catalyst…”
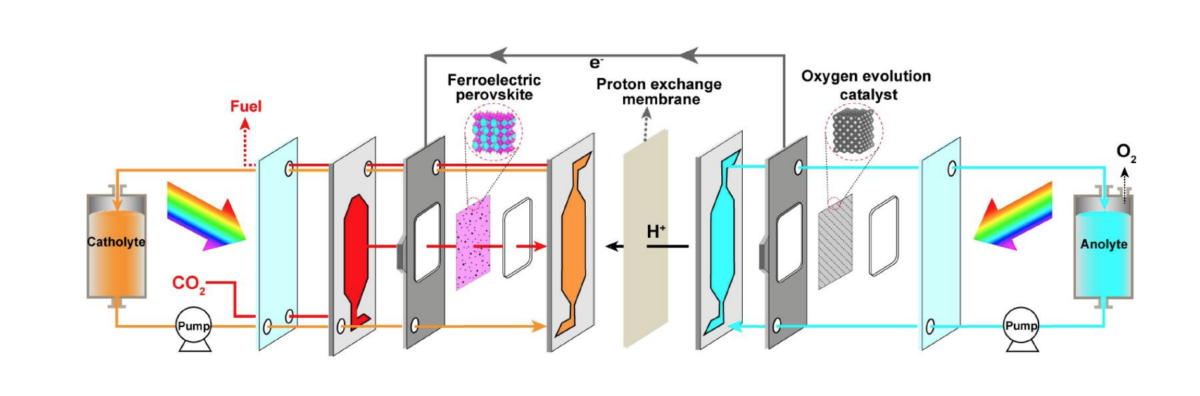
Ma identified a novel ferroelectric, crystalline material that doesn’t have a symmetric structure, “So it has a little bit of polarisation in the structure”, which translates into “an intrinsic and spontaneous electric field” that delivers high-performance photocatalysis.
His Future Fellowship comes under the ARC Discovery Scheme, which aims to promote Australia’s strong capability in fundamental, or ‘blue sky’ research, and to generate new ideas, new jobs, economic growth and enhanced quality of life in Australia, according to the literature.
Ma’s group will use the funding over four years, initially, he says “to generate some high quality papers, scientific breakthroughs and even patent some of the technologies”.
Industry collaborations in place
Because Swinburne focuses heavily on translation of research to industry, Ma’s project team is also already collaborating with GrapheneX, a pioneer in the field of synthesising high-value carbon nanomaterials, from low-cost carbon sources; and with the Clayton Hydrogen Technology Cluster (Clayton H2), one of 15 nationwide renewable energy platforms supported by National Energy Resources Australia.
“We’re working with GrapheneX to treat carbon-dioxide emissions from the biomass lignite [aka brown coal]-mining industry,” says Ma, “and I can foresee that towards the middle of the four-year fellowship, we could partly commercialise some of those technologies.”
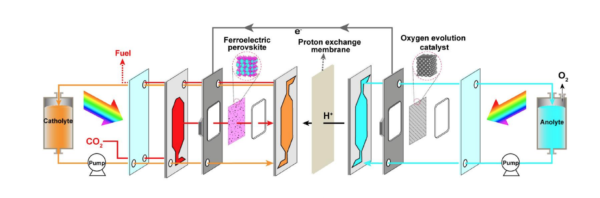
Image: Associate Professor Tianyi Ma/ Swinburne University of Technology
In his six-months-new role at Swinburne, which appointed him Associate Professor at the university’s Centre for Translational Atomaterials, Ma has also already published a paper describing a photocatalytic process to produce hydrogen from seawater, using a novel single-atom platinum catalyst, that yielded solar-to-hydrogen quantum efficiency of 22.2% under LED-550 illumination.
This is “among the best catalysts ever reported” for the solar-to-hydrogen process, said Baohua Jia, Founding Director of the Centre for Translational Atomaterials in February when the paper was released.
Potential pathways for carbon atoms lead back to much needed fuels
Compared to carbon-dioxide splitting, photocatalytic water splitting is a piece of cake, asserts Ma, but he was compelled to pursue CO2 splitting to help reduce greenhouse gas emissions and at the same time produce chemicals that are useful.
The splitting of carbon dioxide, he says, can take more than six potential pathways. It can, for example, be catalysed to form carbon monoxide, formic acid, ethanol or methanol, of which the latter three are important chemical feedstocks.

Image: Associate Professor Tianyi Ma/ Swinburne University of Technology
“We’ve also been doing this photocatalytic reduction of carbon dioxide in an aqueous, or water, solution,” explains Ma, which generates a competing reaction. That is, the water will also take part in the reaction and split out into hydrogen and oxygen.
“So you can never get pure carbon monoxide this way, you always have a monoxide with a little bit of hydrogen, which is what we call syngas — one of the most widely used fuels for residential cooking — there’s a huge market for it.”
Ultimately, his project, as described for Future Fellowship consideration, is expected to “deliver highly efficient photocatalysts and reaction prototypes for carbon dioxide reduction, to relieve the greenhouse effect and accelerate the development of large-scale carbon dioxide utilisation for clean fuels production by making use of abundant and clean solar energy”; the catalysts and techniques “are highly promising for commercialisation and industry-level application”.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








6 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.