Research conducted at Deakin’s Institute for Frontier Materials has discovered a new method called “ball milling” to store gas in a special nanomaterial at room temperature. The method relies on mechanochemical reactions, meaning machinery is used to produce unusual reactions, and the team believes the discovery could help solve the key challenge of hydrogen storage, which is currently a major barrier to uptake.
The breakthrough marks such a departure from accepted wisdom on gas separation and storage that one of the lead researchers, Dr Srikanth Mateti, said he had to repeat his experiment 20 to 30 times before he could truly believe it himself.
“We were so surprised to see this happen, but each time we kept getting the exact same result, it was a eureka moment,” Dr Mateti said.
The special ingredient in the ball milling process is boron nitride powder, which is small but has a large amount of surface area for absorption.
The boron nitride powder is placed into a ball mill – essentially a grinder containing small stainless-steel balls in a chamber – along with the gases that need separating. As the chamber spins at progressively higher speeds, the collision of the balls with the powder and the wall of the chamber triggers a mechanochemical reaction, resulting in gas being absorbed into the powder, the researchers explain.
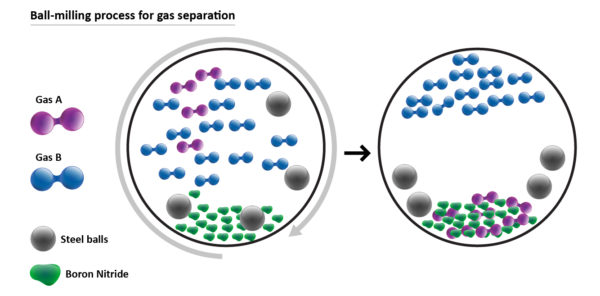
Different types of gases are absorbed into the powdered material at different speeds, meaning they are easily separated from one another through repetition of the process.
“The boron nitride powder can be re-used multiple times to carry out the same gas separation and storage process again and again,” Dr Mateti said.
“There is no waste, the process requires no harsh chemicals and creates no by-products.
“Boron nitride itself is classified as a level-0 chemical, something that is deemed perfectly safe to have in your house. This means you could store hydrogen anywhere and use it whenever it’s needed.”
The researchers described the method as having a “remarkably high gas storage capability.” This, they say, is due to the novel way gas molecules stick to the powder during the ball milling process, which does not break the gas molecules.
The ball-milling gas absorption process consumes 76.8 KJ/s to store and separate 1000L of gases. This at least 90% less than the energy used in the petroleum industry’s current separation process, known as cryogenic distillation.

Image: Deakin University
Once a gas, like hydrogen for instance, is absorbed into the solid state material, it can be transported safely and easily, the researchers say. When the gas is needed, the powder is simply heated in a vacuum to release the gas unchanged.
The breakthrough is the culmination of three decades of work led by Professor Ying (Ian) Chen, the Chair of Nanotechnology at Deakin’s Institute for Frontier Materials, and his team.
“The current way of storing hydrogen is in a high-pressure tank, or by cooling the gas down to a liquid form. Both require large amounts of energy, as well as dangerous processes and chemicals,” Professor Chen said.
“We show there’s mechanochemical alternative… It doesn’t require high pressure or low temperatures, so it would offer a much cheaper and safer way to develop things like hydrogen powered vehicles.”
As it stands, the Deakin team have only been able to test their process on a small scale, separating about two to three litres of material. They are hoping to attract industry support so the discovery can be scaled up to a full pilot.
A provisional patent application for their process has already been submitted, with the breakthrough findings published in the journal Materials Today.
“We need to further validate this method with industry to develop a practical application,” Professor Chen said.
“To move this from the laboratory to a larger industry scale we need to verify that this process is cost saving, more efficient, and quicker than traditional methods of gas separation and storage.”
More specifically, the researchers said for the method to be able to scale, they must perfect the milling process. “There’s a sweet spot in milling which creates the weaker chemical reactions we want – without producing stronger reactions which can destroy the gas molecules,” Professor Chen and Dr Mateti wrote in an accompanying article in The Conversation.
“We will also have to figure out how to get the best storage rate for each material based on milling intensity and pressure of the gases.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








Folks:
Just to save people time… I took a look at the linked Materials Today article, which gives capacities for acetylene and ethylene of around 700 and 1000 cc/gram of boron nitride, respectively. If we assume the volumetric capacity for hydrogen is the same, 1000 cc of hydrogen at STP is 1/24 of a mole or around 80 milligrams of hydrogen gas. The storage capacity would then be about 8 weight%, which is in good agreement with that predicted for specialized boron nitride structures (8.6 wt%) in Shahsavari and Zhuo, Small 2018 v 14 1702863. If this is real, it’s a nice result, somewhat higher storage density than e.g. methylcyclohexane toluene (around 6 wt%) and easier to get the hydrogen out.
And for the authors of this article: please get these numbers from the sources when you write something like this! Without estimates of storage capacity, it’s hard to tell if any given work is important.