Researchers from Victoria’s Monash University claim they have achieved a breakthrough that will bring inexpensive generation of green hydrogen from renewables much closer to reality and make it possible for Australia to further establish its role as a global powerhouse in the generation and export of renewables.
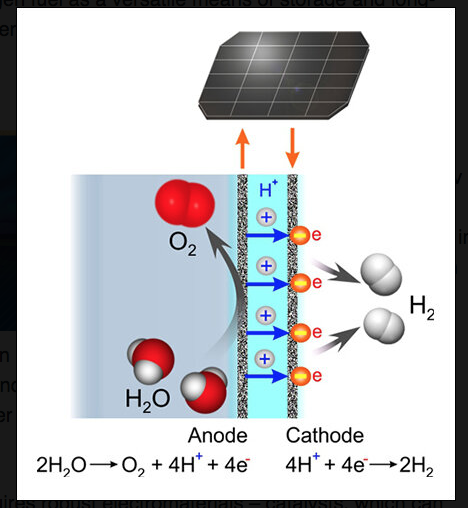
They have developed an electrochemical water splitting method using oxygen evolution catalysts, which are based on non-noble metals and are formed in situ during electrooxidation of acidic water, and exhibit a high stability in operation due to a self-healing mechanism. The researchers believe this method is most likely to be the future of the green hydrogen production.
As noted in their paper published this week in Nature Catalysis, electrolytic water splitting in acidic conditions offers important advantages over that in alkaline systems, but the technological progress is limited by the lack of inexpensive and efficient anode catalysts that can stably operate at a low pH and elevated temperature.
As explained by the lead author of a paper, Alexandr Simonov from the Monash School of Chemistry, the key role in the process is played by robust electromaterials – catalysts, which can accelerate two half-reactions of the water splitting process – the hydrogen evolution and the oxygen evolution reactions.
“Our research team has introduced an intrinsically stable, ‘self-healing’ catalytic system based on earth abundant elements to promote the water electrolysis process in a strongly acidic environment and elevated temperatures,” he says. “The catalyst demonstrates the state-of-the-art activity, and most importantly, exhibits unparalleled stability under a wide range of aggressive, technologically relevant conditions of water splitting.”
The researchers believe that the stability in the operation and the low cost of the developed catalytic system makes it a potentially suitable option for use in the industrial production of green hydrogen fuel by water electrolysis. “The highly disordered mixed metal oxides generated from dissolved cobalt, lead and iron precursors sustain high water oxidation rates at reasonable overpotentials,” the paper states.
Study co-author and ARC Laureate Fellow at the Monash School of Chemistry, Doug MacFarlane said the investigation of water oxidation electrocatalysts is a core theme within the Australian Centre for Electromaterials Science, where he leads the energy program. “It is critically important to the rapidly developing national renewable energy sector,” he said.
Indeed, hydrogen has recently garnered a lot of attention in Australia. The nation’s potential to become the world’s largest producer and exporter of hydrogen powered by solar and wind has been widely reported by national science agency CSIRO, Australian Renewable Energy Agency, Chief Scientist Alan Finkel and even the International Energy Agency, against the backdrop of a rising international demand for hydrogen led by Asian countries. Japan is leading the drive to deploy green hydrogen in its industry and has been described as Australia’s prime export market.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.