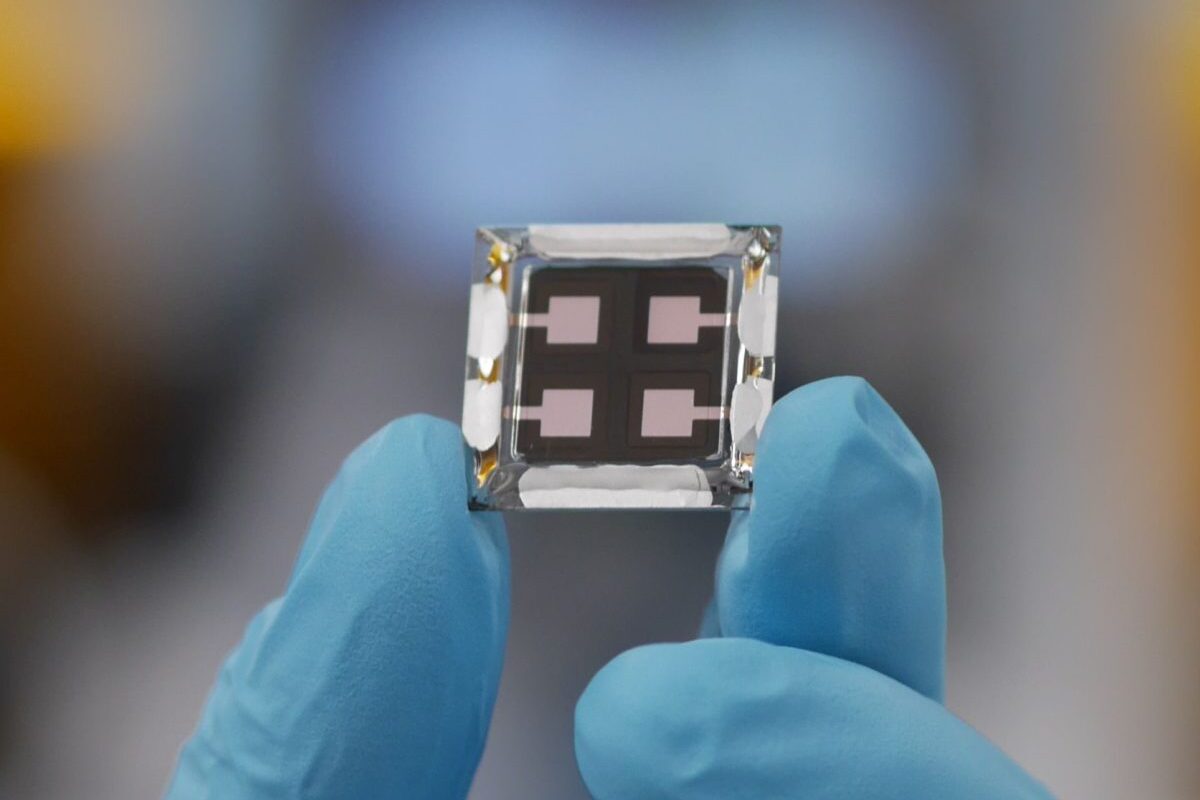
Griffith University researchers have reported a breakthrough in clean hydrogen electrolysis using CoSe2 nanobelts, ultrathin sheets made out of a lattice of cobalt (Co) and selenium (Se), as highly-efficient water splitting electrocatalysts. To fully unleash the power of CoSe2 nanobelts as an electrocatalyst for the oxidation or breakdown of water, the researchers have combined two separate processes.
In a paper published in Nature Communications, the Griffith University researchers describe how they have implemented both ‘Iron (Fe) doping’, replacing some of the cobalt on the nanobelt with iron, and ‘Cobalt (Co) vacancy’, removing some of the cobalt. When applied individually, the two processes improve the nanobelt’s ability to speed up reactions to a small degree but put together their combined effect dramatically increases catalytic activity.
“Our discovery, that by combining these two processes we can push this catalyst to its activity limit, is very exciting. This unlocks not just the catalytic power of CoSe2 nanobelts, but catalysts for all sorts of electrochemical reaction,’’ Dr Yuhai Dou from the Centre for Clean Environment and Energy said.

The thinness of the nanobelts is particularly important to consider when modulating their electronic structure. “The nanobelts are so small they have a thickness of about one nanometre, that’s 50,000 times smaller than the width of a human hair,’’ Dou said. “This thinness hugely increases the surface area and thus reactivity of CoSe2, as only atoms on the surface can react in a solution.”
In alkaline electrolysis, two electrodes are immersed in a liquid alkaline solution. When voltage is applied, water oxidation occurs to produce oxygen at the anode; and water reduction occurs to produce hydrogen at the cathode. Between the two electrodes is a membrane that separates the gases and only allows negatively charged ions of oxygen and hydrogen to pass through. The hydrogen obtained must then be cleaned, dried and if necessary, compressed.
The researchers hope their discovery will advance knowledge in the fields of material science and electrochemistry.“More importantly, with hydrogen being an essential part of the Australian government future energy strategy, this work brings Australian capability to meet the challenge of eco-friendly and efficient hydrogen production a step closer to reality,” Dou said.
Australia’s National Hydrogen Strategy adopted last year aims to establish the nation’s hydrogen industry as a major global player by 2030. The federal strategy, however, remains “technology-neutral”, with both hydrogen produced via electrolysis using solar and wind energy and the one using fossil fuels with “substantial” carbon capture and storage (CCS) in the game. On the state level, governments are stepping up the game delivering their own hydrogen strategies and projects as they seek to unlock the potential of seasonal storage and decarbonize gas networks using green hydrogen in place of natural gas.
While batteries remain a cheaper solution for the decarbonization of transport, clean hydrogen fuel can also do its bit to combat climate change with some projects already in the works. This week alone, Australian resources giant Fortescue Metals Group and Canadian utility ATCO have unveiled plans to build and operate hydrogen refueling facilities for vehicles in Western Australia. The trial of hydrogen-fuelled vehicles hopes to receive funding under the Western Australian government’s Renewable Hydrogen Fund.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.