From pv magazine Global
Among the many different materials and battery chemistries under investigation to fulfil our growing need for energy storage in various applications, zinc-ion has lagged behind others due to challenges in controlling side reactions that both limit reversible charging at the anode, and cause the cathode to fall apart.
Zinc-ion, however, still has the potential for good energy storage performance out of cheap, abundant materials. And at industrial level, could potentially use many of the manufacturing processes already developed for lithium-ion batteries – making them far easier than other materials to scale up. This has been enough to keep scientists interested in working on zinc-ion battery chemistry, and the latest discovery from a group led by the King Abdullah University of Science and Technology in Saudi Arabia (KAUST), could open up several new doorways for them.
Water in salt
Since a lot of these challenges stem from side reactions involving water-based electrolytes, the group worked with a very high salt concentration, limiting the number of ‘free’ water molecules available to the unwanted reactions. The group designed an electrolyte based on zinc salts mixed with a highly concentrated sodium salt, which serves to both suppress reactions with water and increase conductivity, since the mixed electrolyte dissolved more readily than the zinc-salt by itself. “The supporting salt of NaClO4 can efficiently adjust the solvation structure and electronic states of the electrolyte upon increasing the concentrations,” the group explains. “Therefore, the highly concentrated electrolyte shows the scarcity of free water molecules, low viscosity, and high ionic conductivity, which can efficiently prohibit the dissolution of the NVO cathode to enable remarkable cycling stability and high rate performance.”
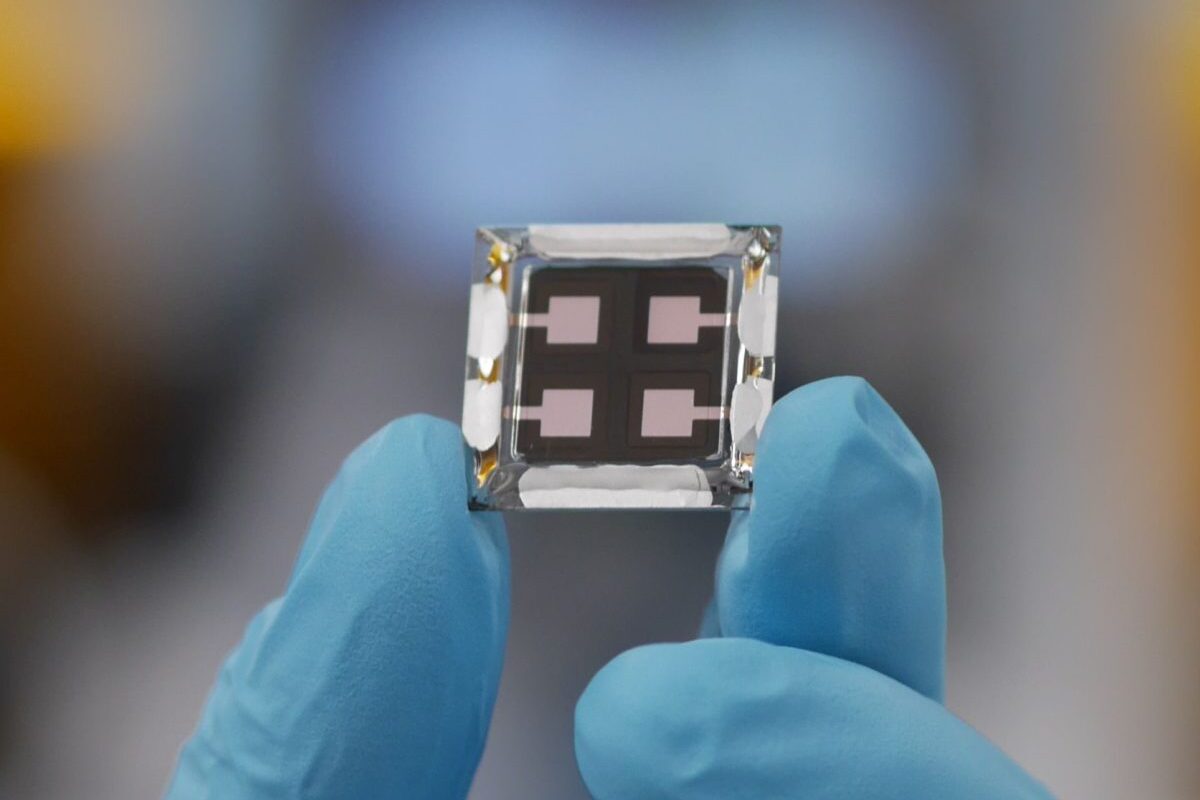
One problem with such electrolytes has been finding suitable cathode materials to work with them, and that can be fabricated cost-effectively. The group worked with a sodium-vanadate material, made using a dissolution-crystallisation process at room temperature, and found this showed strong performance in combination with the electrolyte. “The nanofiber morphology enhances ion diffusion, which ensures faster charge and discharge rates of the aqueous Zn-ion batteries,” said Husam Alshareef, who leads the research group at KAUST. “This combination of electrode and electrolyte potentially solves the shortcomings of conventional aqueous Zn-ion batteries.”
The battery is described in the paper Concentrated dual-cation electrolyte strategy for aqueous zinc-ion batteries, published in Energy and Environmental Science. The group notes that previous ‘water in salt’ electrolytes have often relied on toxic substances, and that it has shown that sodium alone can actually show better ionic conductivity than these, as well as lower cost and reduced toxicity. They also note that in analysing the battery’s performance they discovered a new interphase forming at the anode, which promotes uniform, reversible deposition of the zinc ions as the battery cycles.
With further investigation, the group is confident this battery chemistry could be attractive, at least for stationary storage applications where size/weight is not a pressing concern. “The fundamental understanding and the experimental demonstrations of reversible battery chemistry using a bi-cation electrolyte strategy open a viable route to developing aqueous batteries for emerging electrochemical energy storage applications,” they conclude.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.