University of Adelaide researchers have led an international team to the discovery of a method in which ocean water has been used in a commercial electrolyser to efficiently produce green hydrogen.
“We used seawater as a feedstock without the need for any pre-treatment processes like reverse osmosis desalination, purification, or alkalisation,” Associate Professor Yao Zheng, from the University of Adelaide’s School of Chemical Engineering said. The team simply filtered the seawater, which came from the Huanghai Sea in China, to remove solids and microorganisms.
“The performance of a commercial electrolyser with our catalysts running in seawater is close to the performance of platinum/iridium catalysts running in a feedstock of highly purified deionised water,” Zheng added.
The discovery addresses concerns about water scarcity that have lingered around green hydrogen discussions, with the researchers noting the ocean is an “almost infinite resource,” accounting for 96.5% of the earth’s water reserves, but has proven challenging due to the complexities of the water’s profile.
The team’s solution boils down to adjusting the local reaction environment of the catalyst, which they did by introducing an acid layer on its surface which captured problematic ions and reduced the formations of solids which can block the electrode.
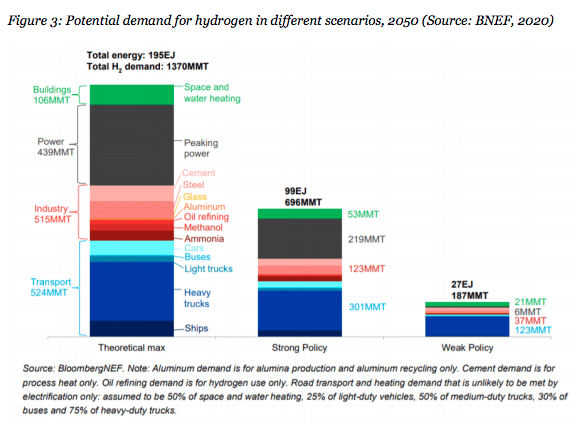
Image: BNEF
The team, consisting of researchers from China’s Tianjin University and Nankai University, and Kent State University in the United States, alongside those from the University of Adelaide, published their research in Nature Energy.
They noted the approach was most practical for regions with long coastlines and abundant sunlight, à la Australia.
The team will now work on scaling up the system by using a larger electrolyser so that it can be used in commercial processes such as hydrogen generation for fuel cells and ammonia synthesis.
Salt in the wound
Given the ocean’s vastness, it is considered a natural feedstock electrolyte. As the researchers note, “direct seawater electrolysis without the purification process and chemical additives is highly attractive and has been investigated for about 40 years.”
The issue – using seawater for electrolysis tends to cause electrode side reactions and corrosion, leading to low efficiency and poor stability of the electrolysis system.
This is because seawater has high concentrations of harmful chlorine ions as well as unwanted positively charged ions like magnesium and calcium ions. Since the pH value of seawater near the cathode increases remarkably during electrolysis, these magnesium and calcium ions can form into massive precipitates like magnesium hydroxide, becoming insoluble solids that can block the electrode.
To address these issues, the team introduced a “hard Lewis acid layer” on the catalyst surface to split water molecules and capture many of the the negatively charged ions surrounding the catalyst. They also found their approach created a strongly alkaline environment, pH 14, which inhibited chlorine production on the catalyst, reducing the formation of these electrode blocking solids.
The team introduced this acid layer on a series of common catalysts to manipulate the local reaction microenvironment, they said, – noting this was a “general strategy that can be applied to different catalysts without the need for specifically engineered catalysts and electrolyser design.”
“With this in situ generated local alkaline environment on a series of Cr2O3-modified catalysts, a substantial activity enhancement was achieved, and simultaneously, the harmful chlorine chemistry and precipitate formation were avoided,” they said.
“The flow-type seawater electrolyser with Cr2O3-modified catalysts afforded a good stability up to 100 h at 500 mA cm−2 and exhibited an industrially required current density of 1.0 A cm−2 at 1.87 V and 60 °C,” the team concluded.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.







Global game changer
Congratulations on achieving this amazing breakthrough …. the generation of hydrogen directly from seawater !!! Perhaps the biggest energy breakthrough this planet was waiting for !!!!
Nice initiative, but it only saves 5kWHr of energy for processing of 1 cubic metre of water into hydrogen and oxygen. It takes 2kWHr to purify groundwater, 7kWHr to purify seawater, and 5,000 kWHr for electrolysis to separate the hydrogen atoms from the oxygen in 1 cu meter of water. The great majority of the energy requirement is still in the splitting of the water molecule, not in the desalination of the water.