For a given volume, ammonia – a molecule made up of three hydrogen atoms and one nitrogen atom – carries about 50% more hydrogen atoms than hydrogen itself.
As ammonia contains only hydrogen and nitrogen, it does not emit carbon dioxide when used. If made using green hydrogen (produced with renewable energy), its production also does not emit carbon dioxide. Therefore, green ammonia could help achieve a net-zero world, particularly as a fuel for long-haul transport and heavy industry.
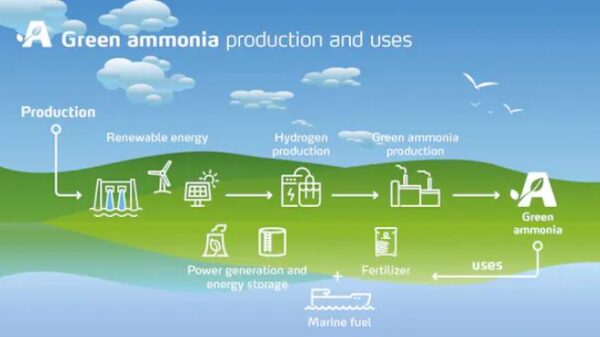
Image: The Davos Agenda/World Economic Forum, CC BY-NC-ND
Australia is well placed to develop a major renewable hydrogen export industry, potentially using green ammonia. Proposed projects include Cape Hardy, Collinsville, Australian Renewable Energy Hub, HyNQ, H2Tas and Gibson Island.
Unfortunately, just because ammonia doesn’t contain carbon, that doesn’t make it good for the environment. It’s a source of nitrogen pollution, which has many damaging environmental impacts. Despite Australia’s natural advantage in producing green ammonia, we ironically have the biggest per capita nitrogen footprint in the world.
Rarely, though, do green ammonia proponents critically assess its environmental sustainability beyond net zero claims.
Exceeding planetary boundaries
The Stockholm Resilience Centre defines nine processes that regulate Earth to support life as we know it. The boundaries of a “safe operating space” have been defined for each process.
You probably won’t be surprised to know climate change is one of the boundaries we have exceeded. You could be forgiven for thinking it has been our biggest impact on the planet. But that “honour” goes to biogeochemical flows of nutrients, mostly as a result of nitrogen fertilisers.
Why? Well it comes back to ammonia.
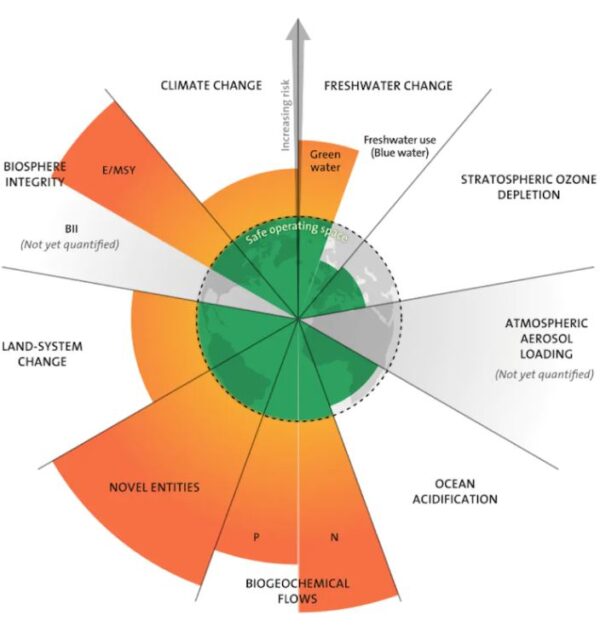
Image: Azote for Stockholm Resilience Centre, based on analysis in Wang-Erlandsson et al 2022, CC BY-NC-ND
Unbalancing the nitrogen cycle
Nitrogen makes up 78% of our atmosphere as dinitrogen gas (N₂). However, in this form it is inaccessible to living organisms.
To support life, nitrogen must be converted into reactive forms such as ammonia. Once it reaches ecosystems, ammonia undergoes a series of chemical transformations. Eventually it breaks up again into N₂, and the cycle can start over again.
For millions of years, the processes in each step of the cycle have been in balance. However, industrial fertiliser production in the 20th century has thrown this cycle out of balance.
On the upside, ammonia has been a miracle chemical for growing food. An estimated 3.5 billion people are being fed thanks to chemical fertilisers. This means almost half of the world’s population would go hungry if not for synthetic ammonia.
The downside is too much reactive nitrogen is ending up in the environment – more than twice as much as the recommended planetary boundary.
In the case of excess nitrogen, crossing its planetary boundary has already had huge consequences. It has led to deterioration of ecosystems, photochemical smog, acid rain and health problems such as respiratory illnesses and cancer, algal blooms leading to fish kills such as the recent one at Menindee Lakes, damage to the Great Barrier Reef, and greenhouse gases much more potent than carbon dioxide.
Producing additional ammonia as a renewable energy carrier could make these problems even worse.
A problem of leakage
Some estimate ammonia supply chains leak it into the environment at rates as high as 6%. However, research is limited. More widely understood natural gas supply chains may provide a ballpark figure of around 2.6%.
Replacing fossil fuels with ammonia for long-haul trucks and shipping might reduce the carbon footprint of transport (which accounts for 37% of total carbon dioxide emissions). Yet, if 2.6% of ammonia leaked from the supply chain, we estimate this could triple the reactive nitrogen flux, further overshooting the planetary boundary. At a 6% leakage rate, it could be four times.
We all know how climate change has been changing our world – and that is at “only” 1.2 times the carbon planetary boundary. Using green ammonia as a renewable energy carrier could have an even greater impact on the nitrogen planetary boundary.
A complement to ammonia
Another renewable energy carrier can be made from green hydrogen: methanol. It’s a molecule of four hydrogen atoms and single carbon and oxygen atoms.
Like ammonia, methanol can be used as a fuel. It could replace petrochemical feedstocks used in industrial processes and manufacturing. Our research shows methanol could also enable biotechnology to better integrate with industrial processes.
More significantly, methanol doesn’t affect the nitrogen cycle. As long as it is made using a renewable source of carbon, such as carbon dioxide from direct air capture, there is a net zero impact on the environment. International Energy Agency projections show the United States has a greater emphasis on green methanol, while Australia and Europe are more focused on green ammonia.
Environmental sustainability means more than net zero. In the case of green ammonia, holistic thinking is needed so we don’t solve one problem only to make another worse.
Authors: , PhD Candidate; , Senior Researcher, Australian Centre for Water and Environmental Biotechnology, The University of Queensland
This article first appeared in The Conversation and is republished here with permission.
The views and opinions expressed in this article are the author’s own, and do not necessarily reflect those held by pv magazine.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








When I was working on Australia’s fuel standards in the early 2000’s there was quite a lot of interest both in methanol and ethanol as esters and as fuel additives. It was the potential for water table pollution from methanol derivatives, and effective lobbying from ethanol interests, that resulted in the present ethanol landscape.