South Australia Flinders University researchers, in collaboration with Griffith University, have published findings into the potential of aqueous zinc-ion batteries as a more sustainable energy storage technology alternative to lithium-ion batteries.
Published in a September 2024 issue of Energy Storage Materials journal, the research paper is titled Converting a low-cost industrial polymer into organic cathodes for high mass-loading aqueous zinc-ion batteries.
The FU Jia Research Group lead, College of Science and Engineering Associate Professor in Chemistry and Nanotech Researcher Zhongfan Jia said aqueous zinc-ion batteries could have real-world applications, from electric vehicles to portable electronic devices, as polymer AZIBs using organic cathodes are low-cost, simple to produce and potentially biodegradable.
AZIBs can also solve supply-chain issues of strategic metals including lithium and cobalt availability and the cost of spent batteries needing to be recycled.
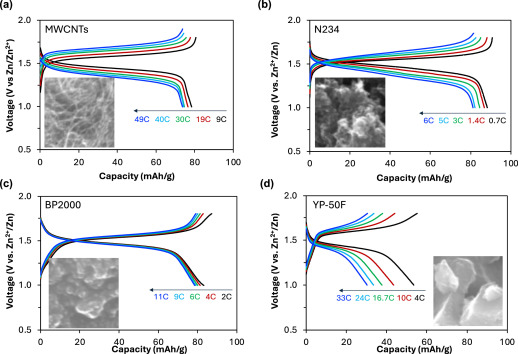
Image: Flinders University
“AZIBs stand out because of the much higher abundance of zinc in the earth’s crust (ten times more than lithium), and their low toxicity and high safety,” Jia said.
AZIBs usually use zinc metal as an anode and inorganic or organic compounds as a cathode. While substantial work has been devoted to improving the stability of zinc anodes, high-performing cathodes are needed and remain a major challenge.
“Our research is building conductivity using nitroxide radical polymer cathodes made from cheap commercial polymer and optimised the battery performance using low-cost additives,” Jia said.
“The work re-evaluates the use of high redox potential nitroxide radical polymers cathodes in AZIBs and produced the highest mass loading so far.”

Image: Flinders University
The study is led by Flinders master student Nanduni Gamage and postdoc fellow Dr Yanlin Shi who have developed a lab-made pouch battery using scaled-up polymer (at an approximate cost of $20 / kg), a non-fluoro Zn(ClO4)2 electrolyte, and BP 2000 carbon black ($1 / kg) without binder to provide a capacity of nearly 70 mAh g-1 and a middle discharge voltage of 1.4 V.
With a mass loading of 50 mg cm-2, the pouch battery had a capacity of 60 mAh, which easily powered a small electric fan and a model car.
Collaborators in the study include Dr Jesús Santos-Peña, from the Université Paris Est Creteil CNRS in France who worked with other experts from the Flinders University Institute for Nanoscale Science and Technology.

Image: Flinders University
The team has also reecently developed organic radical/K dual-ion batteries, a technique that can also relieve dependence on lithium-ion batteries.
The research is being supported by Australian Research Council and the French-Australian International Research Network on Conversion and Energy Storage (IRN-FACES) funding, and the Australian National Fabrication Facility (ANFF) SA node for supporting the electroanalytical and electrochemical synthesis labs at Flinders University.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








3 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.