University of Adelaide scientists have developed a new dry electrode preparation technique that has been found to boost energy density, reduce battery degradation and improve overall stability of aqueous zinc–iodine batteries.
Aqueous zinc–iodine batteries are considered safer, more sustainable, and more affordable than lithium-ion systems but they still fall short in overall performance.
Led by Professor Shizhang Qiao from the university’s School of Chemical Engineering, the team has created an electrode fabrication process that avoids the conventional wet processing of iodine cathodes that leads to iodine sublimation, low active material loading, poor electrode compacted density, and significant polyiodide shuttle effects, all of which limit the energy density and practicality of these systems.
“We mixed active materials as dry powders and rolled them into thick, self-supporting electrodes,” Qiao said. “At the same time, we added a small amount of a simple chemical, called 1,3,5-trioxane, to the electrolyte, which turns into a flexible protective film on the zinc surface during charging.”
“This film keeps zinc from forming sharp dendrites – needle-like structures that can form on the surface of the zinc anode during charging and discharging – that can short the battery.”
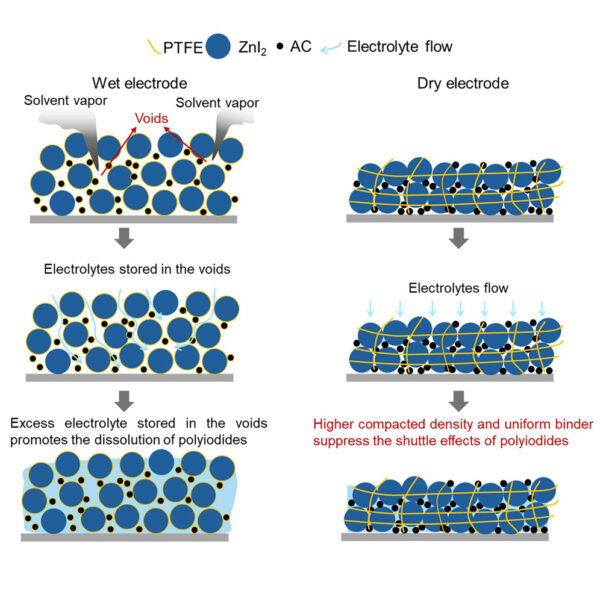
The dry electrode preparation technique resulted in “remarkable” cycling stability and record-high mass-loading iodine cathodes, research associate Han Wu said.
“The new technique for electrode preparation resulted in record-high loading of 100 mg of active material per cm2,” Wu said.
“After charging the pouch cells we made that use the new electrodes, they retained 88.6% of their capacity after 750 cycles and coin cells kept nearly 99.8% capacity after 500 cycles.”
The researchers said high iodine loading and a robust zinc interface mean much more energy can be stored in each battery at a lower weight and cost, an advance that could speed up the real-world adoption of zinc–iodine technology for large-scale and grid-level storage applications.
“The new technology will benefit energy storage providers, especially for renewable integration and grid balancing, who will gain lower-cost, safer, long-lasting batteries,” Qiao said.
“Industries needing large, stable energy banks, for example, utilities and microgrids, could adopt this technology sooner.”
The research team is now looking to develop the technology further to expand its capabilities.
“Production of the electrodes could be scaled up by using to reel-to-reel manufacturing,” Qiao said.
“By optimising lighter current collectors and reducing excess electrolyte, the overall system energy density could be doubled from around 45 Wh kg to around 90 Wh kg.”
“We will also test the performance of other halogen chemistries such as bromine systems, using the same dry-process approach.”
The team published their results in the journal Joule.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.







By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.